
Towards a scientific-based farming of sea urchins: First steps in the cultivation of Diadema setosum, Diadema savignyi and Mesocentrotus nudus
Keywords
Diadema savignyi · Diadema setosum · Larval culture · Larval development · Mesocentrotus nudus
1. Introduction
The global trend of aquaculture development gaining importance in total fish supply has remained uninterrupted. In 2012, farmed food fish contributed 42.2% of the total 158 million tonnes of fish produced by capture fisheries (including for non-food uses) and aquaculture. This compares with only 13.4% in 1990 and 25.7% in 2000 (FAO, 2020). In 2016, global aquaculture production (including aquatic plants) was 110.2 million tonnes, with the first-sale value estimated at USD 243.5 billion. In 2018, the aquaculture production volume was about 938,500 tonnes of aquatic animals (USD 6.8 billion) such as turtles, sea cucumbers, sea urchins, frogs, and edible jellyfish (FAO, 2018).
Sea urchins are not only a source of high protein delicacy food but are also a potential source of biological active substances used for medical purposes (Rahman, Arshad, & Yusoff, 2014). Harvesting of natural sea urchins has possibly reached its limit, and thus, it is likely that the global production of sea urchin roe from wild fisheries will decline (Rahman et al., 2014). In recent years, a central circle of countries comprising producers and suppliers of products from sea urchins to the world market has been developed (Stefansson, Kristinsson, Ziemer, & Hannon, 2017). For further sustainable development, scientific studies of the biological principles of reproduction of mariculture objects must be introduced.
Countries in the Asia-Pacific region with a high population are experiencing problems of a lack of protein-rich food. Sustainable development of coastal countries can be established by linking their economies with sea resources (Figure 1).
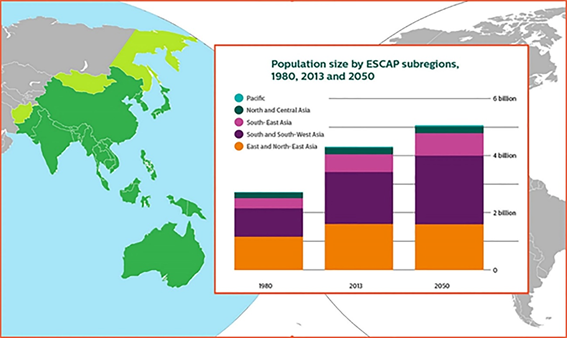
Resources from the sea in the region are not only fish but also invertebrates, which diversify our food resources. In recent years, the problem of overfishing of aquatic biologic resources has become acute. The solution to this problem requires strict compliance with the catch rates regulations and the development and application of methods for artificial cultivation of aquatic objects. Science-based approaches can enhance farming techniques, and one such approach is the development of coastal mariculture to reproduce commercial species using sophisticated technology. Along with the creation of marine aquaculture farms using traditional objects, like oysters, sea urchins, sea algae, et cetera, it is necessary to study the reproductive characteristics of other common species. Marine objects may have been the earliest food objects for humans. Evidence is provided by the so-called kitchen heaps, which were found at the sites of ancient people located on the sea coast. Analysis of the spectrum of food habits of ancient man shows that sea urchins made up a significant part of the diet of people. Since ancient times, sea urchin gonads are culinary delicacies in many parts of the world. Sea urchin roe is considered as a prized delicacy in Asian, Mediterranean and Western Hemisphere countries and has long been a luxury food in Japan. Sea urchin mariculture, together with other farmed animals, is of growing importance in such a situation.
The goal of the study was to present comparative data on reproductive characters of three common species of sea urchins. Marine aquaculture is one way to meet the growing demand for healthy marine food. To date, there are no cost-effective technologies for growing sea urchins in aquariums. Some countries obtained wild seed materials for further cultivation up to commercial size in suitable water areas (McBride, 2005). In both cases, knowledge of the embryonic and larval development of target species of sea urchins may be in demand.
Choosing species for possible cultivation, we drew attention to sea urchins, which are common and in consumer demand, although poorly investigated in terms of the biology of their embryonal and larval development. Our study focused on two species of Diadema, which are widely distributed on the coast of Viet Nam and many other areas in the Indo-Pacific. These species are in demand, and we witnessed this in the vicinity of the Viet Nam towns, like a Nha Trang.
Mesocentrotus nudus was selected as it is especially loved in Japan, the primary consumer of sea urchin uni. This is also a common species on the coast of Russia’s Primorsky Region. While Strongylocentrotus intermedius has been cultivated for quite some time in mariculture farms in Primorye, Mesocentrotus nudus has not yet been maricultured.
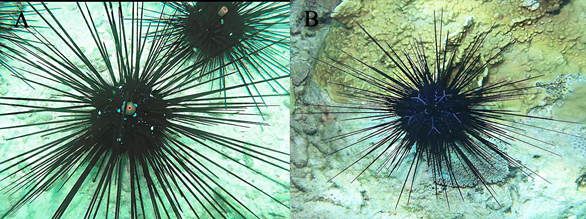
Diadema setosum (Figure 2(A)) and D. savignyi (Figure 2(B)) are common species in the South China Sea, and they inhabit similar biotopes (Liao & Clark, 1995). In Viet Nam’s coastal waters, these species are distributed around rocky and stony substrata at the upper layers of the sublittoral zone. Sea urchins with long thin spines can cause harmful stings, and they have so far retained their numbers and form dense mixed groups on stony bottoms, and climbing into deep crevices between large stones.
The larvae of Diadema are remarkable in planktonic populations. Those of D. setosum were described by Mortensen (1921, 1931, & 1937) and more recently by Rahman, Yusoff, & Arshad (2015) from Malaysia and Dautov and Dautova (2016) from Viet Nam.
The distinctive features of adult D. setosum and D. savignyi are obvious (Figure 2). D. setosum has five white spots at the aboral side, orange rim around the anus, and chains of blue points on the test surface along the ambulacra (Figure 2(A)). On the other hand, D. savignyi has a set of characteristic blue lines at the aboral side, and a dark blue rim around the anus (Figure 2(B)). The morphology of their larvae (Dautov & Dautova, 2016) and the longevity of the larval development were investigated (Rahman et al., 2015). Here we present the original account of the larval common structure of D. savignyi, and for comparison, we present data on the development of the similar sea urchin D. setosum, both of which are from the Nha Trang Bay of the South China Sea.
Sea urchin Mesocentrotus nudus (A. Agassiz, 1864) inhabits the Japan and Yellow seas. Outwardly, this sea urchin looks somewhat like diadematids – black and armed with spines about 3-4 cm in length (Figure 3). M. nudus larval development was investigated in Japan waters (Kawamura, 1970). However, the area of the species is wider in the Asia-Pacific. The data on the larval development of M. nudus collected at the coast of Primorye region (Peter the Great Bay, the Sea of Japan) is presented here, for comparison.

Our interest in Diadema setosum, D. savignyi and Mesocentrotus nudus was initiated by the lack of information about larval development and schedule of the development of these species. At the same time, these are common species in the region – D. setosum and D. savignyi in the tropical Indo-Pacific, and Mesocentrotus nudus in the Japan and Yellow seas. These species are consumed by the citizens of Japan and local aboriginal communities in Southeast Asia countries. Moreover, Mesocentrotus nudus known as the former Strongylocentrotus nudus is very popular in Japan.
2. Methodology
2.1 Cultivation of larvae in the laboratory
2.1.1 Sea urchins of genus Diadema (Diadema setosum (Leske, 1778) and Diadema savignyi (Audouin, 1829))
The investigation of the larval development of Diadema setosum and D. savignyi was carried out in April-July, from 2014 to 2016. The work consisted of the following steps:
- Collecting adult sea urchins. Adult and mature sea urchins of Diadema setosum and Diadema savignyi with a test diameter of about 60-70 mm were collected in Nha Trang Bay (South China Sea) by skin divers. In the laboratory, sea urchins were maintained for 2-3 days in tanks with running seawater (t° = 28-29°C) until spawned. About 50-60 specimens of both species were used. They were unfed in the laboratory.
- Water preparation. Filtered seawater (FSW) was obtained by filtrating through 0.4 µm filters. The volume of FSW used depends on the frequency FSW in jars containing the larvae (twice a day).
- Obtaining gametes. To obtain gametes, long spines were trimmed off and sea urchins were placed mouth upward, and gonopores downward on glass cups with FSW. Spawning was induced by injecting 0.2-0.5 ml of 0.55M KCl solution through the peristome (Iwata & Fukase, 1964). Spawning females remained in the glass cups, shedding eggs into the FSW. Sperm from males were collected in Petri dishes (dry sperm) and placed in a refrigerator until used. After shedding gametes, sea urchins were released back to the sea. For artificial fertilization, eggs from 3-4 females and semen from 3-4 males were used.
- Shaded eggs from 3-4 females (approximately 10-12 million eggs) were washed three times with FSW, placed in a thin layer on the bottom of a 2-litre cup containing FSW, and fertilized by adding 5-6 drops of a freshly prepared suspension of spermatozoa (20 µl dry semen diluted in 60 ml of FSW). Samples of the eggs were taken periodically after insemination to confirm successful fertilization by checking under a compound microscope. The zygotes developed their fertilization membrane at 20-40 minutes after fertilization. Further, the zygotes were washed three times in FSW and left in the same cup until the swimming blastula stage.
- Cultivation of larvae. The next day, larvae in the stage of swimming blastula were transferred to 4-litre glass cans with a density of 2-3 larvae per ml, in which they were cultured according to Strathmann (1987), with permanent agitation of seawater until the larvae had settled and metamorphosed. The temperature in the room was 27-28°C. By reverse siphon, about 90% of the water was changed twice a day – in the morning at 6:00 and in the evening at 18:00. Preliminary experiments indicated that changing seawater twice a day was required for normal development of larvae at 27-28°C. The larvae were fed on the unicellular algae Nannochloropsis oculata. Food was added during every water changing with an approximate concentration of 2000-3000 cells/ml. Several larval cultures were initiated during our investigation – one in 2012, six in 2013, and six in 2014 for D. setosum; and eight in 2015, and three in 2016 for D. savignyi. Each culture consisted of 12 4-litre cans with a density of 2-3 larvae per 1 ml.
- Analysis and photoregistration. About 20 larvae were measured and photographed daily in the early stages (from 1 to 10 days). During 10 to 40 days of cultivation and before larval settlement, 2-5 larvae were taken for analysis two times a week. They were examined, photographed and returned to culture dishes. Live embryos and larvae were observed under a compound microscope. Larvae were photographed with a digital camera by putting the camera lens to a microscope eyepiece with an ocular micrometre. The size of eggs, embryos, blastulae and larvae were measured from the photo images. Sizes are given as mean value (MV) ± standard deviation (SD). In the late plutei of D. setosum and D. savignyi, the entire larvae with arm lengths greater than 2 mm did not fit within the field of view even with the smallest magnification lens. Their arm lengths were measured using panoramic photos with images combined and enhanced in Adobe Photoshop CS6 8.1 6.3.9600.17415 (Adobe Systems Software Ireland Ltd., 6 Riverwalk, Naas Road 24, Dublin, Ireland).
2.1.2 Sea urchins of genus Mesocentrotus (Mesocentrotus nudus (A. Agassiz, 1864))
For the investigation, the adult sea urchins Mesocentrotus nudus (A. Agassiz, 1864) from Vostok Bay (Sea of Japan) were used.
- Collecting adult sea urchins. Skin-divers collected mature sea urchins at the reproductive season (July-September 2007). In the laboratory, sea urchins were placed in baths with running seawater until the experiment. During the investigation, 60 specimens of nudus were used with diameter of 60-80 mm. The sea urchins were returned to the sea after shading of gametes.
- Preparing FSW. Seawater was filtered through three layers of fine gravel and sterilized by water running under ultraviolet (UV) rays from quartz lamps (Dautov & Kashenko, 2008).
- Obtaining the gametes. Gametes were obtained by standard methods. Sea urchins were placed mouth up on to glasses with FSW and spawning was induced by injection of 0.2 ml of 0.55M KCl solution through the peristome (Iwata & Fukase, 1964). Spawning animals remained in the glass caps, shading gametes into FSW. Petri dishes with sperm from males were placed in a refrigerator until required.
- Eggs from three females and sperm from three males were used for artificial fertilization. Shaded eggs were washed several times in FSW and placed in a thin layer at the bottom of a 4-litre cup with FSW. Five to six drops of a freshly prepared suspension of spermatozoa that looked somewhat opalescent were added to eggs. The success of fertilization was checked under a compound microscope. The zygotes developed their fertilization membranes at 20-30 minutes after insemination. Further, the zygotes were washed three times in FSW and left in the same cup until the swimming blastula stage.
- Cultivation of larvae. The next day, larvae in the stage of swimming blastula were transferred to 5-litre glass jars with a density of 5–6 larvae per ml. After the larvae had attained the 4-armed pluteus, they were fed with equal parts of three algae: Nannochloris maculata, Isochrysis galbana and Chaetoceros muelleri. Each alga made up one-third of the total concentration of 5,000-7,000 cells per ml, i.e. approximately 1,700-2,300 cells per ml. At the 6-armed pluteus stage (6-7 days after fertilization (a.f.)), we added two more algae Phaeodactylum tricornutum and Dunaliella salina to the feed mixture. The total number of algae remained the same – 5,000-7,000 cells per ml, and all five algae had an equal proportion – i.e. the concentration of alga species in the feed mixture was 1,000-1,400 cells per ml.
- Analysis and Photoregistration. A Reichert Polivar microscope with a Canon PowerShot S40 digital camera and a Leica DC 150 camera adapter were used to observe and register the development of the larvae. For measurements and observation, 20 larvae were taken from cultural jars the next day.
For image processing and preparation of the pictures, Adobe Photoshop CS6 8.1 6.3.9600.17415 (Adobe Systems Software Ireland Ltd., 6 Riverwalk, Naas Road 24, Dublin, Ireland) was used.
3. Results
3.1 Larvae of the sea urchins family Diadematidae Gray, 1855
3.1.1 Diadema setosum (Leske, 1778)
Diadema setosum (Leske, 1778) developed from small isolecithal eggs with a diameter of 84.2 ± 3.1 μm (n = 25). Embryonic development took about 6.5-7.0 h and finished when a blastula left the fertilization envelope and became a larva (Dautov & Dautova, 2016).
Young rounded blastulae (Figure 4(A)), which swam around their longitudinal axis, gradually changed body shape. The late blastula just before gastrulation became elongated and had a narrow anterior tip (length 95.0 ± 1.7 μm, width 88.2 ± 1.7 μm (n = 14)), and they developed the first pigment cells. The first mesenchymal cells were visible in the narrow blastocoel. Then the blastoderm became thinner, the blastocoel more spacious, and the sclerenchyma produced primary spicules (Figure 4(B)). The blastula became a prism at the beginning of the invagination (Figure 4(C)). The width of the young prism (106.3 ± 2.0 μm) was greater than its length (88.2 ± 1.7 μm (n = 21)). The first pigment cells had appeared at this stage. At 23 h a.f., a prism developed; at 44 h a.f., a pluteus with one pair of arms had appeared (Figure 4(D)); at 45 h of development, plutei had two pairs of arms. The pigment cells coloured the pluteus of D. setosum dark red (Figure 4(E)). Six-day old pluteus had grown its arms (Figure 4(F)). The postoral arms of plutei grew to 1900 μm or more during further development (Figure 4(G)). Metamorphosis took place over 40-45 days. At this time, five primary ambulacral podia were visible within the larval body. The duration of metamorphosis from the moment of larval settlement until the juvenile sea urchins began to move along the bottom was 40-60 min. The diameter of the newly metamorphosed juvenile sea urchins was about 500 μm (Figure 4(H)).
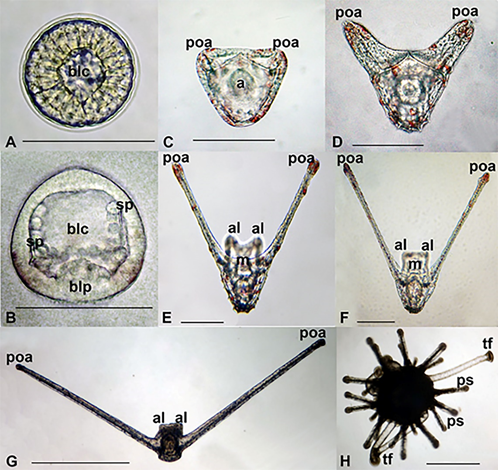
D. setosum refers to sea urchins with small eggs. Fecundity of these urchins was about 4-5 million eggs. A high natural loss occurred during the cultivation of sea urchin larvae. Some embryos developed abnormally and did not turn into larvae. The development of larvae occurred at different rates. Many larvae became retarded in development so much so that even after 40-50 days of development, they remained at the stage of early pluteus. The percentage of metamorphosed was 0.0042% (0.0014% – 0.0052%), and this was calculated as the number of juvenile sea urchins to the total number of larvae in the culture.
3.1.2 Diadema savignyi (Audouin, 1829)
The gametes obtained from the females Diadema savignyi had diameters of 63.8-94.9 µm with an average value of 79.24 ± 6.37 µm (n=149). Immediately after hatching, the blastulae were spherical and 100 µm in diameter and usually swam around the longitudinal axis in the upper layer of water (Figure 5(A)). After about 15–18 h. a.f., the spherical blastulae became slightly elongated. Shortly before gastrulation began, the wall of the blastula’s body became thinner, the blastocoel became more spacious and two groups of mesenchymal cells that formed two primary triaxial spicules were visible (Figure 5(B)). The larvae became prisms during gastrulation. The blastopore, located on the vegetal pole of the larva, shifted to the ventral side of larvae. Prisms had a blastopore, which became the anus, and after the prism stage was completed, the mouth had appeared (Figure 5(C)).
Development of the D. savignyi pluteus consisted of several stages. The first stage, immediately after the prism, was the pluteus stage with one pair of arms (Figure 5(D)). At this stage, pluteus already had a complete larval skeletal basket and digestive tract with mouth, oesophagus, stomach, and intestine with anus. A few hours later, the second pair of arms visualized in the larva (Figure 5(E)). Approximately up to the 8th day of development, the larvae had hand growth processes, and the postoral arms grew much faster than the anterolateral ones (Figure 5(E)).
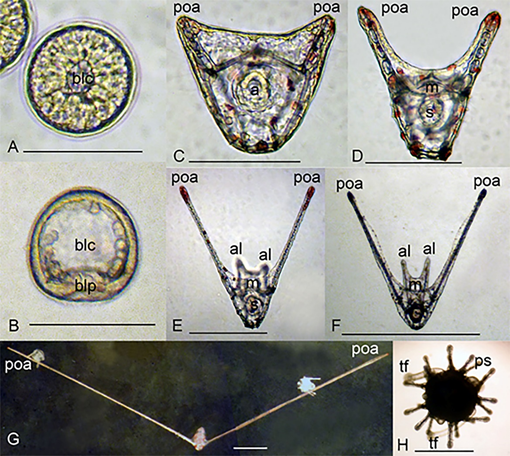
During the development, the appearance of the larva changed. In addition to a substantial elongation of postoral arms, changes occurred in the proportions of the larval body which became shorter and more oval. Anterolateral arms gradually became so short that they were difficult to see (Figure 5(G)). Nevertheless, the rods of anterolateral arms were retained in the latest larvae. The larva was then settled down and the metamorphosis completed. The young sea urchin immediately after metamorphosis had five tube feet and fifteen primary spines (Figure 5(H)).
At the end of larval culture, we had about 30-50 juveniles from 1% of the eggs from three females.
3.2 Larvae of the sea urchins fam. Strongylocentrotidae Gregory, 1900
3.2.1 Mesocentrotus nudus (A. Agassiz, 1864)
The eggs obtained from female Mesocentrotus nudus had a diameter of 98.0±7.8 µm. First larval stage of this sea urchin was spherical blastula (Figure 6(A)). Blastula left the fertilization envelope and began its larval life. Blastulas swam around the anteroposterior axis of the body. Gastrulation began at second day a.f. (Figure 6(B)). Gastrulas swam around the longitudinal axis. From the bottom of the blastocoel (at the posterior end of the larval body), primary mesenchymal cells originate. They produced first skeletal ossicles.
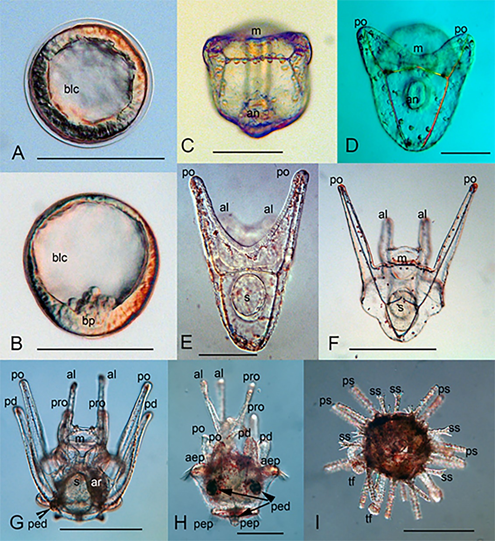
At three days a.f., larvae became a prism. The archenteron reached the ectoderm at the ventral side, and the mouth appeared in a late prism (Figure 6(C)). At the blastocoel, one can see the skeletogenic cells and primary spicules. Four-day old late prism had the first pair of larval arms – postoral ones. The archenteron came to contact with the ectoderm, and the mouth had formed (Figure 6(C, D)).
Young pluteus (4-5 days a.f.) had differentiated digestive tracts, larval skeletal baskets, and attained anterolateral arms (Figure 6(E)). Late four-armed pluteus had well-developed postoral and anterolateral arms (Figure 6(F)).
Full-developed pluteus of Mesocentrotus nudus had eight arms (Figure 6(G)) – two postoral, two anterolateral, two posterodorsal, and two preoral ones. At the left side of the larvae, the rudiments of the adult sea urchin had developed (Figure 6(G)). The rudiment of primary pedicellaria can be recognized at the posterior part of the larval body. This larva swam and fed on phytoplankton.
Larva attained upper and lower epaulettes and three pedicellaria through further development (Figure 6(H)). The ciliary band of the pluteus is the main organ for swimming and feeding. At the end of the larval life, the larvae of regular sea urchins attained epaulettes – short segments of the ciliary band, which served only for swimming. Pluteus before metamorphosis had well-developed epaulettes and three pedicellariae. One can see the resorption of the larval arms (Figure 6(H)).
The metamorphosis included the reduction and collapse of the larval structures and appearance of the adult structures – test, primary and secondary spines, and tube feet (Figure 6(I)).
All larval development from the fertilization to metamorphosis took about 30-31 days at 21°C. At the end of cultivation, we had about a hundred juveniles from one larval culture.
The results of our investigation are summarized in Table 1. For comparison, we proposed the results of our preliminary studies on the dynamics of larval development in several common species of sea urchins from the Nha Trang Bay (Table 2).
| Species | Diadema setosum (26-28°C) |
Diadema savignyi (26-28°C) |
Mesocentrotus nudus (21°C) |
| Time | |||
| 0 hour | Zygote | Zygote | Zygote |
| 6-7 hours | Blastula | Blastula | – |
| 18-19 hours | gastrula | gastrula | – |
| 2 days | 2 armed pluteus | 2 armed pluteus | gastrula |
| 3 days | 4 armed pluteus | 4 armed pluteus | prism |
| 4 days | 4 armed pluteus | 4 armed pluteus | 2 armed pluteus |
| 6 days | 4 armed pluteus | 4 armed pluteus | 4 armed pluteus |
| 9 days | 4 armed pluteus | 4 armed pluteus | 6 armed pluteus |
| 18 days | 4 armed pluteus | 4 armed pluteus | 8 armed pluteus |
| 30 days | 4 armed pluteus | 4 armed pluteus | Pluteus (epaulettes, pedicellaria) |
| 31 days | 4 armed pluteus | 4 armed pluteus | Juvenile |
| 44 days | Juvenile | 4armed pluteus | – |
| 47 days | – | Juvenile | – |
Table 1. Time-developmental stage of Diadema savignyi, D. setosum, and Mesocentrotus nudus (T°C).
Table 1 shows that the sea urchins Diadema have a longer larval stage compared to Mesocentrotus nudus. The temperature of development is an essential factor. We reared M. nudus at 21 ± 1 ° С. Considering that this sea urchin lives in the temperate waters of the Japanese and Yellow Seas, it requires a lower temperature compared to the conditions for keeping the culture of Diadema sea urchin larvae.
| Species (egg size, µm) Stage |
D. setosum |
D. savignyi |
P. maculata |
T. pileolus |
T. gratilla |
| Blastula | 6.5 h | 7-12 h | 17 h | 12 h | 19 h |
| Prism | 23 h | 23-31 h | 27 h | 42 h | 35-40 h |
| 2-arm pluteus | 44 h | 48 h | 42 h | 60 h | 50 h |
| 4-arm pluteus | 45 h | 50 h | 3 d | 3 d | 3 d |
| 6-arm pluteus | – | – | 11 d | 12 d | 12 d |
| 8-arm pluteus | – | – | 17 d | 19-24 d | 20-22 d |
| Metamorphosis | 44 d | 47-50 d | No | 30-33 d | 30-33 d |
Table 2. Some dimensions and temporal parameters of embryonic and larval development of Diadema setosum, D. savignyi, Pseudoboletia maculata, Toxopneustes pileolus, and Tripneustes gratilla (personal observations, unpublished) (27 ± 1°С).
Table 2 shows the results of preliminary studies of maintaining cultures of larvae of common species of sea urchins from the Nha Trang Bay. It is seen that the popular mariculture object Tripneustes gratilla completed larval development and settled in 30-33 days. Toxopneustes pileolus also settled and underwent metamorphosis in 30-33 days. Another common species from the Nha Trang Bay, Pseudoboletia maculata may be the promising object of the mariculture. This sea urchin grew to an 8-armed pluteus in 17 days. Unfortunately, we were not able to bring the culture of the larvae of this sea urchin to metamorphosis.
4. Discussion
McEdward and Miner (2001) provide a classification of the reproduction modes of all echinoderm species studied at the time. In a comprehensive review by Pearse and Cameron (1991), based on an analysis of the literature data, the general structure of echinopluteus of certain sea urchin orders is given. In all species considered in our investigation, the planktotrophic larva echinopluteus developed. Diadema had a modified larva, in which only two pairs of arms formed. The common structure of the larvae of two related species investigated was so similar that they were difficult to distinguish from each other. Sometimes they are called two-armed (Huggett et al., 2005), since in the latest stages of their development in the late Diadema plutei, only a pair of postoral arms grew out of the two pairs of larval arms. The second pair of arms – anterolateral ones noticeably growing slower, although it did not completely disappear until the end of larval development. The skeletal rods of the anterolateral arms were preserved until the latest larval stages, so the Diadema plutei remained four-armed until metamorphosis. Larvae of D. savignyi and D. setosum differed from D. antillarum (Eckert, 1998). Late pluteus of D. antillarum had thicker arms and therefore can be easily distinguished from the pluteus of D. setosum and D. savignyi.
Some similarity in common characters of adult urchins, i.e., black colour, size of the test, length of spines (3-4 cm in Mesocentrotus nudus, about 30-40 cm in Diadema setosum and D. savignyi) bears some convergence. The ontogenesis and larval structure differed significantly.
Comparing larval morphology of Mesocentrotus nudus and Diadema setosum and D. savignyi shows weak resemblance. In all mentioned species, a blastula was the first larval stage. It dissolved the fertilization membrane and began its pelagic life. Blastula of Diadema had a thick wall and relatively small blastocoel. In M. nudus, blastula had relatively thin blastoderm.
The larvae of D. setosum and D. savignyi are practically indistinguishable in size and colour. Some differences in the structure of the larvae are visible only at the latest stages of development. The late pluteus of each species had clear and specific features. Plutei of Diadema have long arms (in Diadema setosum – about 2300 µm, in Diadema savignyi – about 5500 µm) whereas the length of the arms of Mesocentrotus nudus did not reach 1000 µm.
Pedicellaria are formed in some late plutei of both species of Diadema, and this pedicellariae is species-specific (Coppard & Campbell, 2006; Rahman et al., 2015; Dautov, personal observation, unpublished). All late plutei Mesocentrotus nudus have three pedicellariae.
Pedicellaria in regular sea urchins are an obligatory attribute of the late stages of the pluteus. They are situated on the right side of the pluteus body and somehow participate in the process of settlement and temporary attachment to the substratum. The absence of pedicellaria in the late pluteus can speak of the abnormal development of the larva (Burke, 1980). In larvae of Diadema, probably, these formations are optional.
The differences in the length of the postoral arms of the plutei of Diadema savignyi and D. setosum cannot be the result of differences in the growing conditions, since larvae of both species were kept in the same conditions.
The difference in the developmental duration of Diadema sea urchins between our cultures and the data obtained by Rahman et al. (2014) on D. setosum in Malaysia and Eckert (1998) on D. antillarum, can indicate a difference in the genetics level of these species and the conditions of the cultivation and feeding of larvae. Perhaps, it will be possible to modify the scheme of cultivation of larvae, which will affect the time of larval development. This will be the content of further experiments with these interesting marine animals.
Until early 1990, Strongylocentrotus intermedius and Mesocentrotus nudus abounded off the coast of Primorye region. With the beginning of 2000, the situation changed. Natural seafood sharply went up in price on the world market, which led, in particular, to an increase in interest in the oriental way of life and nutrition. Demand and prices for sea urchins and their caviar increased. Now when the S. intermedius has been caught almost entirely and become noticeably rare, interest to Mesocentrotus nudus as a fishing target has grown significantly. M. nudus inhabits the same places that S. intermedius. Larval morphology of the M. nudus differs from the specific morphology of the larvae of genus Strongylocentrotus members. Young S. intermedius pluteus have an outstretched posterior tip of the body (Naidenko, 1983). In Mesocentrotus nudus, the posterior tip of the body is rounded. There are differences between late plutei. Late pluteus Strongylocentrotus intermedius has a pointed posterior end of the body and greenish body colour. In the Mesocentrotus nudus, late pluteus has a rounded posterior end of the body and a dark reddish colour.
We can compare the temporal characteristics of the larval development of the species studied. In all cases, the results obtained can be useful for developing schemes for obtaining juvenile stages that can be used as a seed.
The world’s most edible species of sea urchins are: Centrostephanus rodgersii, Echinometra sp., Evechinus chloroticus, Heliocidaris erythrogramma, Loxechinus albus, Lytechinus variegatus, Paracentrotus lividus, Psammechinus miliaris, Salmacis sphaeroides, Strongylocentrotus droebachiensis, Strongylocentrotus franciscanus, Strongylocentrotus intermedius, Strongylocentrotus (Mesocentrotus) nudus (the most popular in Japan ~44%), Strongylocentrotus purpuratus, and Tripneustes gratilla. The last species – Tripneustes gratilla – lives in Viet Nam and can be used as a potential object for marine farming. This species is now harvested from natural populations, and there is a fear of overfishing. In the near future, one will certainly need to restore its population.
Diadema setosum and D. savignyi are not in the list mentioned previously. Nonetheless, in Malaysia, D. setosum is cultivated (Rahman et al., 2015) and is in demand among customers of seafood restaurants.
5. Some conclusions
For sea urchins proposed by us, embryonic and larval development is described, with a detailed description of all stages.
Due to its specific structure, the larvae of the genus Diadema are sometimes called two-armed. The development time to metamorphosis in the Diadema can vary, which can be explained by the characteristics of laboratory cultures (especially at high temperature) and the properties of local populations. The whole development takes about 45-46 days at 26-27°C.
Mesocentrotus nudus have a typical 8-handed pluteus, which differs slightly from the morphology of larvae of sea urchin genus Strongylocentrotus. Development of the M. nudus takes about 30 days at 21°C. Cultivation at moderate temperatures reduces the cost of preparing seawater.
Despite of the small percentage of juvenile specimens (0.001% or lower), the proposed cultivation scheme may well be used to obtain seed that can be further grown in suitable controlled water areas.
One can compare the common structure of the larvae from obtained culture with the figures from the article. This gives hope that research can be used to improve people’s lives.
Acknowledgement
This work was supported by the Asia-Pacific Network for Global Change Research through its Climate Adaptation Framework (grant number CAF2016-RR08-CMY-Dautova). We are grateful to the Institute of Oceanography and its staff for their kind help, and the facilities and infrastructure provided.
References
- Burke, R.D. (1980). Development of pedicellariae in the pluteus larva of Lytechinus pictus (Echinodermata: Echinoidea). Canadian Journal of Zoology, 58:1674–1682.
- Coppard S.E., & Campbell A.C. (2006). Systematic significance of tridentate pedicellariae in the echinoid genera Diadema and Echinotrix. Invertebrate Biology, 125(4): 363-378. doi:10.1111/j.1744-7410.2006.00068.x
- Dautov, S.S., & Dautova, T.N. (2016). The larvae of Diadema setosum (Leske, 1778) (Camarodonta: Diadematidae) from South China Sea. Invertebrate Reproduction & Development, 60 (4): 290–296. doi:10.1080/07924259.2016.1238411
- Dautov, S.S., & Kashenko, S.D. (2008). Development of the Sand Dollar Scaphechinus mirabilis. Russian Journal of Marine Biology, 34(6): 415–420. doi:10.1134/S1063074008060114
- Eckert, G.L. (1998). Larval development, Growth and Morphology of the Sea Urchin Diadema antillarum. Bulletin of Marine Science, 63(2): 443–451.
- FAO. (2018). The state of world fisheries and aquaculture 2018 – Meeting the sustainable development goals. Rome. Licence: CC BY-NC-SA 3.0 IGO.
- Huggett, M.J., Catherine, K.K., Williamson, J.E., & Steinberg, P.D. (2005). Larval development and metamorphosis of the Australian diadematids sea urchin Centrostephanus rodgesii. Invertebrate Reproduction and Development, 47(3): 197–204. doi:10.1080/07924259.2005.9652160
- Iwata, K.S. & Fukase, H. (1964). Comparison of discharge of the gametes by three artificial means in sea urchins. Biological Journal of Okayama University, 10, 57–64.
- Liao, Y., & Clark, A.M. (1995). The echinoderms of Southern China. XXIII Pl, Beijing: Science Press.
- Kawamura, K. (1970). On the development of the planktonic larvae of Japanese sea urchins Strongylocentrotus intermedius and S. nudus. Scientific reports of Hokkaido Fisheries Experimental Station, 12, 25–32 (in Japanese with English abstract).
- McBride, S.C. (2005). Sea Urchin Aquaculture. American Fisheries Society Symposium 46, 179–208.
- McEdward, L., & Miner, B.G. (2001). Larval and life-cycle patterns in echinoderms. Canadian Journal of Zoology, 79, 1125–1170. doi:10.1139/z00-218
- Mortensen, T. (1921). Studies on the development and larval forms of echinoderms. G.E.C. Gad, Copenhagen. 261 pp.
- Mortensen, T. (1931). Contributions to the study of development and larval forms of echinoderms. I–II. Mem. de L’Academie Royale des Sciences et des Letters de Danemark. Copenhague. Section des Sciences. 9me série, IV (1), 1–39.
- Mortensen, T. (1937). Contributions to the study of development and larval forms of echinoderms. III. Mem. de L’Academie Royale des Sciences et des Letters de Danemark. Copenhague. Section des Sciences. 9me série, VII (1), 1–65.
- Pearse, J.S. & Cameron, R.A. (1991). Echinodermata: Echinoidea. In Ed. A.C. Giese, J.S. Pearse and V.B. Pearse (Eds), Reproduction of Marine Invertebrates. Vol. VI. Echinodermata and Lophophorates. 513–662 p. The Boxwood Press. Pacific Grove, California.
- Naidenko, T.Х. (1983). Laboratory cultivation of the sea urchin Strongylocentrotus intermedius. The Soviet Journal of Marine Biology, 9(1), 46–51.
- Rahman, A.M., Arshad, A., & Yusoff, F.M. (2014, July). Sea (Echinodermata: Echinoidea): Their biology, Culture and Bioactive Compounds. International Conference on Agricultural, Ecological and Medical Sciences (AEMS-2014). London (United Kingdom).
- Rahman, A.M., Yusoff, F.M., & Arshad, A. (2015). Embryonic, larval and juvenile development of tropical sea urchin, Diadema setosum. Iranian Journal of Fisheries Sciences, 14(2), 409-424.
- Stefansson, G., Kristinsson, H., Ziemer, N., Hannon, C. (2017). Markets for sea urchins: A review of global supply and markets. Iceland Food and Biotech R&D. Matis. October 2017.
- Strathmann, M.F. (1987). Reproduction and development of marine invertebrates from the northern Pacific coast: Data and methods for the study of eggs, embryos and larvae. Seattle: University of Washington Press.
- FAO. (2014). The state of world fisheries and aquaculture: Opportunities and challenges. Retrieved from http://www.fao.org/3/a-i3720e.pdf
- FAO. (2020). The state of world fisheries and aquaculture 2020. Retrieved from http://www.fao.org/state-of-fisheries-aquaculture