
A case study on regional arsenic sources and its distribution in Mekong River groundwater
Keywords
HIGHLIGHTS
- The arsenic concentrations in household tube wells were investigated in Cambodia (KMH) and Lao (LAO), where the arsenic contamination (exceeding the WHO drinking water guidelines) observed in KHM is approximately 30 times higher than that in the LAO region.
- The physiochemical properties of the water samples demonstrated a more oxidising water in LAO (high nitrate with absence of ammonia), lower iron content, and lower pH than that in KHM. This suggests that the arsenic in the LAO study area could potentially be attributed to a secondary reabsorption phase. Different topographical redox conditions could control the arsenic mechanisms in the groundwater system.
- The highest arsenic concentrations were discovered in shallow wells at depths of approximately 40 m, in KHM. The concurrent presence of reducing water chemistry suggests that processes such as reductive dissolution and potentially bacterial sulphate reduction could have substantial effects on the mobility of arsenic. These mechanisms are particularly relevant due to the presence of young organic matter which serves as a carbon source and an electron donor for microorganisms. This dissolved organic carbon (DOC) is reported to rapidly recharge into the groundwater through ponds or clay windows.
1. INTRODUCTION
The amount of exploitable water resources in the world barely makes up 0.3% of its total (Kılıç, 2020). As the global population continues to expand, there is a significant increase in the demand for clean water across various sectors (Salehi, 2022). Clean water is essential not only for maintaining our health but also for supporting agriculture, energy production, navigation, recreation, and manufacturing activities (Biswas & Tortajada, 2018). Arsenic ranks as the twentieth most abundant element on the Earth is naturally ubiquitous within the ecosystems. The presence of arsenic (As) in groundwater, which is utilised as a source of drinking water, is linked to various health issues (Phan et al., 2016). These include skin abnormalities, the development of lung, liver, and kidney cancers, cardiovascular disorders, and the potential impact on the well-being of a significant global population (Abernathy et al., 1999; Barringer & Reilly, 2013; Fatoki & Badmus, 2022; Hong, Song, & Chung, 2014). Thereby, the provisional guideline established by the World Health Organisation (WHO) suggests a maximum allowable limit of 10 µg/L arsenic content in drinking water. Despite its threat to human health, it is well known that arsenic contamination in Southeast Asia is featured by contaminated sediments especially in Holocene deltaic and organic-rich surface sediments. In those areas, arsenic level in groundwater can exceeded values hundreds of times higher than the concentration recommended for drinking water (Hanh, Kim, Bang, & Hoa, 2011; Ishii, Tamura, & Ben, 2021; Luu, Sthiannopkao, & Kim, 2009; Penny, 2006).
Arsenic in groundwater can be released through (a) oxidation of aquifer arsenical pyrite and other arsenic-bearing sulphide minerals, (b) reductive dissolution of arsenic-rich Fe(III) oxyhydroxides and Al-hydroxides, and (c) exchange of adsorbed arsenic with other competitive anions, such as phosphate, bicarbonate and silicate (McCarty, Hanh, & Kim, 2011). Nevertheless, the reductive dissolutions of arsenic-rich Fe(III) oxyhydroxides and/or Al-hydroxides were widely accepted to be the main mechanism of direct arsenic mobilisation. The mechanisms responsible for its release can vary depending on the specific geological and hydrological conditions, such as redox reactions (Herath, Vithanage, Bundschuh, Maity, & Bhattacharya, 2016), microbial activity (Ko, Lee, & Kim, 2019), dissolution from minerals (Robinson et al., 2009; Welch & Stollenwerk, 2003), etc. It is important to note that the factors that regulate these mechanisms also vary depending on the characteristic of bedrock aquifer, recharges rate, flow processes, groundwater levels, etc. (Lipfert, Reeve, Sidle, & Marvinney, 2006; Peel et al., 2022). During intense and prolonged low flow, the decrease in the water table’s level is anticipated to amplify the oxidation process of sulphides containing arsenic within the unsaturated zone. Furthermore, reduced groundwater flow enhances geochemically evolved arsenic-rich groundwater consequently boost the release of arsenic through reductive dissolution and alkali desorption (Bondu, Cloutier, Rosa, & Benzaazoua, 2016).
Southeast Asia has been identified as a significant “climate change hotspot,” (Yusuf, 2010). The primary climatic risks affecting the countries include flooding, drought, flash flooding in the mountainous parts of the country, windstorms, and large-scale farmland erosion. Situated within latitudes of 10–15° north of the equator, both Cambodia and Lao region experiences a tropical monsoon climate or inter-annual variations in climate (annual dry and wet seasons) due to the influence of El Niño Southern Oscillation aka., monsoon season from May to mid-October, and a dry season from mid-October to April. During the wet season, flooding accompanies overflow of the Mekong River and its tributaries, which is triggered by tropical storms, intense monsoonal rainfall, and the runoff originating from the northern mountains (Kazama et al., 2012; Koem & Tantanee, 2022; Phy et al., 2022). Cambodia, predominantly impacted by floods and droughts, achieved the eighth position out of 193 nations in 2014 Maplecroft’s Climate Change Vulnerability Index, a composite measure that assesses a country’s sensitivity to extreme weather events (Davies et al., 2015). Climate change has the potential to increase the frequency and intensity of flooding and/or drought, both of which already cause severe hardship to communities. The impact of extreme climate recharge events on the fate of arsenic is currently a subject of debate and is greatly contingent upon the characteristics of the bedrock aquifer, which in turn influence the susceptibility of groundwater.
To date, the scarcity of data concerning the household arsenic levels in the high-risk arsenic zones further poses a significant challenge in assessing the potential evolution of the resource quality. The mechanism responsible for regulating groundwater arsenic concentrations in arsenic high-risk zone can be heterogeneous and site-specific underlying to its intricate interactions with geological formations, hydrological conditions, and chemical processes. The purpose of this study is identifying spatial arsenic in the sub-region groundwater of Mekong River area which is heavily affected by arsenic and typical of many circum-Himalayan shallow aquifers. The arsenic release mechanism is investigated through the interactions between water bodies. The study on the regional arsenic contamination levels is important for the government integrate regional groundwater arsenic risk reduction policy planning.
2. METHODOLOGY
2.1. Study area
The design of the study area was based on the location of Mekong River Basin, specifically in Lao (LAO) and Cambodia (KHM). The Sekong river were selected as the control site. After the release of COVID-19 travel restriction, sampling was conducted in households nearby the Mekong River during May and August 2022. The study map is presented as in Figure 1 where groundwater samples were collected 2–3 km from the river basin. The study area was located from (14°77′ to 15°10′ N, 105°76′ to 106°83′ E) for the Lao sampling site whereas (11°24′ to 11°33′ N, 105°07′ to 105°14′ E) for KHM sampling site. A total of forty-four tube-well water samples located at Mekong River sub-region were collected from household or community tube in May and August of 2022. Laos is situated more to the north, as Figure 1 shows, in the upstream of the Mekong’s passage through Lao PDR; while the Sekong river originated in the Central Highlands of Vietnam is one of the most important Mekong tributaries. It flows downstream through Laos and merge with Mekong River in Cambodia, contributing ten percent of the water flow to the Mekong River. Samples in Sekong river area were collected at Laos before its merge into Mekong River in Cambodia.
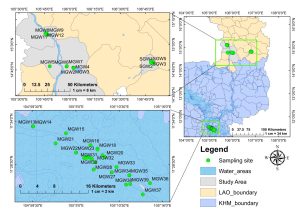
The study areas along the Mekong River located from the southern parts of Lao PDR to Cambodia are floodplain areas. In Cambodia, a significant portion of the land consists of a floodplain, with approximately 85% of the country’s land located within the lower Mekong basin. Tube-well water is used by the communities for drinking, cooking, and household consumption as well as for irrigation. The depth of tube wells tested varied from 10 to 60 m. Meanwhile, the sampling area of Cambodia is located at a lower topographic gradient than in Laos. The average temperature of the study area is around 26 to 30 °C with the highest temperature recorded in May (Tsujimoto, Ohta, Aida, Tamakawa, & So Im, 2018). A study of seasonal influences on groundwater arsenic near to our sampling locations did not show much evidence of seasonal-scale recharge of the Bassac River to aquifer except one site where arsenic concentration was increased from 1.3 µg/L to 8.6 µg/L due to wet season recharge processes (Tweed et al., 2020). The seasonal fluctuation was not essential, and therefore was not included in this study.
2.2. Sample collection and analysis
Groundwater samples were collected from 44 households in May and August of 2022. Ultra-clean sampling protocols were used to prevent contamination of samples. Ancillary parameters, such as dissolved oxygen, pH, temperature, salinity, total dissolved solid and conductivity were recorded in situ using a multi-parameter probe. Filtered surface water samples were collected using a syringe filter connected to disposable PTFE filter membrane (Advantec®;, 0.45 µm). At each site, duplicate samples were collected in polyethylene bottles for analysis (Yu et al., 2015). The water samples then preserved with high-purity nitric acid, HNO3 (0.1% v/v), and stored at 4 °C until they were measured with inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Elemental X7) in the Gwangju Institute of Science and Technology.
2.3. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 20 for Windows. A principal component analysis (PCA) was applied to extract the components of the variables of parameters in groundwater (Bartlett, 1954; Cattell, 1966; Kaiser, 1960) to understand the factor on the release of arsenic in Kandal and Prey Veng groundwater, where high range of arsenic in groundwater were observed.
3. RESULTS AND DISCUSSION
3.1. Physicochemical properties
The physicochemical properties of groundwater collected were shown in Table 1. In summary, KHM groundwater showed an average dissolved oxygen (DO) up to 4.7 mg/L, electric conductivity (EC) 738.6 µS/cm, total dissolved solid (TDS) 370.1 ppm and salinity 0.2 ppt. The average temperature and pH were 32.5 °C and 7.3, respectively. As compared with the results reported on 2008, the average temperature was increased by 4.3 °C (Park et al., 2011). The results of the in-situ analysis (Table 1) showed that the acidity level of all the water samples were relatively neutral (pH = 6.8 to 7.8). The overall TDS and EC value observed in this study was slightly lower than the data collected in Kahe catchment area during wet season. Both TDS and EC have been regarded as a major contribution by increase in runoff during monsoonal precipitation (R2 = 0.84) (Lwimbo, Komakech, & Muzuka, 2019). Nitrate in groundwaters in KHM study area is mostly below the detection, except site MGW 13, 25, and 27 where ammonia concentration was ranged from 0 to 0.7 mg/L. Other sites showed high ammonia concentration compared to nitrate. Such high ammonia concentration potentially anthropogenic sources resulting reducing water in the groundwater thus highlighted the infiltration of water from surface to groundwater.
| Parameters | Mekong River | Sekong River | Chanpiwat et al. (2011) | |||
|---|---|---|---|---|---|---|
| Average | Range (min–max) | Average | Range (min–max) | Average | Range (min–max) | |
| DO (mg/L) | LAO: 2.8 | LAO: 1.9–4.0 | 5 | 2.9–8.6 | – | – |
| KHM: 4.7 | KHM: 3.5–5.6 | |||||
| Temp. (°C) | LAO: 28.0 | LAO: 26.8–28.8 | 29.3 | 29.2–30.1 | – | – |
| KHM: 32.5 | KHM: 29.8–35.8 | |||||
| pH | LAO: 6.5 | LAO: 5.7–7.2 | 6.9 | 5.8–6.9 | 6.58 | 3.54–7.37 |
| KHM: 7.3 | KHM: 6.8–7.8 | |||||
| EC (µS/cm) | LAO: 462.6 | LAO: 116.3–744 | 342.4 | 183.1–509 | 358.27 | 132–1520 |
| KHM: 738.6 | KHM: 228.7–1787 | |||||
| TDS (ppm) | LAO: 295.8 | LAO: 74.4–476.2 | 219.1 | 117.1–325.8 | 358.27 | 27.8–1520.0 |
| KHM: 370.1 | KHM: 114.5–901.3 | |||||
| Salinity (ppt) | LAO: 0.1 | LAO: 0–0.3 | 0.1 | 0–0.2 | 0.31 | 0–0.95 |
| KHM: 0.2 | KHM: 0–0.5 | |||||
| Nitrate (mg/L) | LAO: 4.0 | LAO: 0–10.5 | 9.6 | 1–17 | – | – |
| KHM: 5.9 | KHM: 5.1–7.5 | |||||
| Ammonia (mg/L) | LAO: 0.6 | LAO: 0–0.6 | 0 | 0 | – | – |
| KHM: 4.1 | KHM: 0–28.1 | |||||
In comparison, LAO groundwater showed lower averaged DO, EC, TDS, salinity, temperature, and pH compared to KHM groundwater. The groundwater physicochemical characteristics demonstrated the distinct in water bodies between LAO and KHM groundwater. Other reason could be due to the dilution after rainfall events which means the groundwater in LAO region could be mostly of unconfined aquifer. It is important to note that higher nitrate concentration was observed in all LAO and Sekong groundwater as compared to ammonia concentration which contrasts with that observed in the KHM groundwater. This phenomenon could be attributed to the topographic gradient effect, as Cambodia is situated at a lower topographic gradient in comparison to LAO. Other than that, the water catchment area demonstrates that the study area in KHM was predominantly covered by water, in contrast to the LAO and Sekong study areas where dry land was more prevalent (Figure 1). Though the samples were collected from similar groundwater depth range, the topographic gradient is steeper in KHM (Cambodia) than in LAO (Laos), resulting in the groundwater bodies in KHM groundwater to be more reducing compared to the LAO groundwater bodies. Variance in topographic gradient contributes to differing hydrogeological conditions and redox potentials, subsequently influence the geochemical characteristics of the respective groundwater (Lei, Wagner, & Fohrer, 2021).
3.2. Arsenic concentration in groundwater
The average total arsenic concentration was 81.36 ppb, 6.1 ppb and 7.0 ppb in KHM (n = 27), LAO (n = 12), and Sekong (n = 5), respectively. The sampling site where arsenic concentration exceeds the WHO guideline (>10 µg/L) was mainly found in the KHM study area i.e., Kdei Kandal village (MGW 13), Kvea Aem village (MGW 16, 17, 18, and 19), Khsom village (MGW 22 and 23), Stueng village (MGW 38), and Chrouy Dang village (MGW 39). The percentage of sample sites that showed high arsenic contamination was about 33% (9 out of 27 sampling sites). In LAO and Sekong, three sites out of twelve and one site out of five showed arsenic (>10 µg/L), however not exceed 20 µg/L. The range of arsenic concentration observed in KHM is approximately 30 times higher than those in LAO and Sekong area, Figure 2. High arsenic concentration observed in groundwater has been observed in countries such as Argentina, Chile, Mexico, China, and Hungary, and more recently in West Bengal (India), Bangladesh and Vietnam (Smedley & Kinniburgh, 2002).
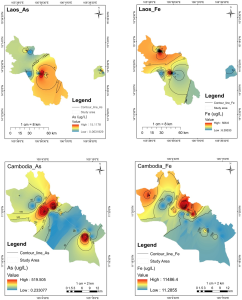
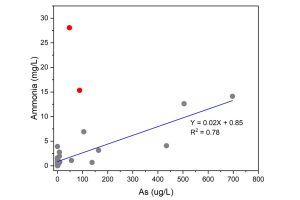
In Cambodia, high arsenic was reported to be contributed by strongly reducing aquifers specifically from arsenic-bearing alluvium (Chanpiwat et al., 2011). The reducing aquifers were further proved through our study where high correlation was observed between ammonia and arsenic (Figure 3). In KHM, water nitrate ranged from 5.1–7.5 mg/L (n = 4) where most samples showed nitrate concentration lower than the detection limit while high ammonia (ranged from 0–28.1 mg/L) was accompanied by high arsenic (except for site MGW 17 and 18). In site MGW 17, the ammonia concentration was 28.1 and arsenic concentration was 50 ppb while for site MGW 18, 15.3 mg/L ammonia was accompanied by 90 ppb of arsenic. This suggests the potential anthropogenic sources of nitrogen infiltrating into the groundwater, and it’s possible that these two sites are associated with an unconfined aquifer. In contrast to KHM, the water nitrogen content is LAO is mostly in oxidised form (Table 1) where ammonia could barely detect. This proposed that the mechanism controlling the release of arsenic might be different between KHM and LAO.
Other than that, slightly lower pH was observed in LAO water bodies compared to the KHM groundwater (Table 1). Mineral surfaces tend to acquire a negative charge under alkaline conditions; therefore, a higher pH (7 to 8.5) leads to a reduction in the sorption of arsenic oxyanions, ultimately resulting in an increased likelihood of arsenic release (Ravenscroft, Brammer, & Richards, 2009). This could be the main mechanism underlying in KHM study area. Under anoxic conditions, the introduction of oxygen triggers the oxidation of arsenic-containing minerals, resulting in significant alterations to water chemistry. The oxidative dissolution of these minerals releases iron, sulphur and arsenic. However, under near-neutral pH conditions, arsenic tends to precipitate with iron, adhere to iron (hydr)oxides, or integrate into secondary oxide phases. In a study (Battistel, Stolze, Muniruzzaman, & Rolle, 2021), it was found that 40% of the released arsenic through oxidative dissolution was reabsorbed into a newly formed iron-arsenate phase. This phase formed a coating on the mineral surface, which further limited the extent of dissolution reactions. The presence of a secondary reabsorption phase might explain the comparatively low levels of arsenic concentration in the LAO study area.
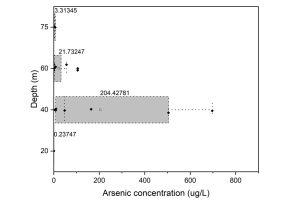
Sampling site that yields high arsenic was also associated with shallow groundwater zone with depth between 35–65 m (Figure 4) which agree with the finding in the study of Buschmann, Berg, Stengel, and Sampson (2007) where up to >1000 µg/L of arsenic was reported between 25 and 45 m depth. However, similar trend was not observed in either LAO or Sekong groundwater samples. Rapid weathering of arsenic-bearing rocks in the upper Himalayan catchments is transported by large rivers and deposited in the low-lying, young alluvial floodplains (Polya et al., 2005). The Mekong River conveys approximately 160 million tons of sediment annually that ultimately reaches the South China Sea (Ta, Nguyen, Tateishi, Kobayashi, & Saito, 2001). The mechanisms responsible for arsenic release was reported to be varied by the sources of organic carbon. Extensive groundwater pumping likely caused the lateral intrusion of transporting the young organic carbon to greater depth such as in Bengali (∼80 m) and the Red river (∼120 m) (van Geen et al., 2013). Study of arsenic release in shallow groundwater using tritium and radiocarbon data also demonstrated that rapid recharge through ponds or clay windows transport the young, surface derived organic matter into groundwater under natural flow conditions (without groundwater abstraction) can reach up to 44 m (Lawson, Polya, Boyce, Bryant, & Ballentine, 2016; Richards et al., 2017). Given these number of recharges, relatively young labile organic matter was reported to play an important role in (a) microbial-mediated reductive processes and (b) reduction of Fe(III) oxyhydroxides resulting in elevated concentrations of arsenic under reducing conditions that develop in young (Quaternary) Holocene alluvial aquifers in this region (Lawson et al., 2013; Polizzotto, Kocar, Benner, Sampson, & Fendorf, 2008).
3.3. Factor analysis on arsenic distribution in KHM Mekong River sub-region groundwater
To provide better insight into the mechanisms of arsenic in KHM groundwater, parameter associations were performed by principal component analysis (PCA) as shown in Figure 5. Variables with a correlation matrix (r) of less than 0.3 were eliminated from the data interpretation. The first three factors were adopted as shown in Table 2, which explain 74.4% of the variance expressed by the data matrix. After the factors were extracted, a rotation of the factorial axis was performed (varimax algorithm). The factor loadings showed that factor 1 (43.5% of the variance) has factor loadings > ±0.5 for salinity, cation, anion, TDS, EC, and Mg. This factor represents the major cations and anions resulting from water-rock interactions in the aquifer. Factor 2 (19.5% of the variance) has factor loadings > ±0.5 for Fe, Ba, As, and Ca. Obviously, this factor reveal that elevated As levels occurred under reducing conditions, i.e. high Fe(II). Lastly, factor 3 (11.4% of the variance) has factor loadings > ±0.5 only for Mn and pH. In this study, the concentration of Fe in LAO water bodies was about 20 times lower than those found in KHM water bodies. An increase in As was found together with high Fe (Guo et al., 2016) as proposed, where reductive dissolution of iron (III) oxyhydroxides play an important role leading to arsenic release from aquifer sediments under anoxic conditions. Yet the fundamental mechanism might begin with bacterial sulphate reduction (BSR) (Moore et al., 2023). Similar mechanisms were report by Kocar et al. (2008) whereby under reducing conditions, As-bearing Fe (hydr)oxides drives the reductive mobilisation and release As to the aquifer in the Mekong River.
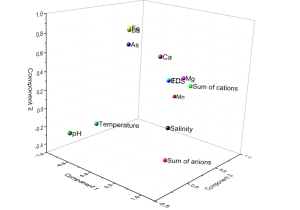
| Parameters | Rotated Component Coefficients | |||
|---|---|---|---|---|
| Component 1 | Component 2 | Component 3 | Communalities | |
| Salinity | 0.832 | 0.263 | ||
| Sum of anions | 0.819 | −0.349 | 0.654 | |
| Sum of cations | 0.810 | 0.353 | 0.358 | 0.930 |
| TDS | 0.791 | 0.481 | 0.931 | |
| EC | 0.789 | 0.482 | 0.794 | |
| Mg | 0.666 | 0.415 | 0.358 | 0.909 |
| Fe | 0.903 | 0.736 | ||
| Ba | 0.884 | 0.543 | ||
| As | 0.736 | 0.744 | ||
| Ca | 0.616 | 0.693 | 0.816 | |
| Mn | 0.833 | 0.793 | ||
| pH | −0.785 | 0.697 | ||
| Temperature | −0.431 | 0.867 | ||
4. CONCLUSION
Overall, the study highlights the physicochemical properties of groundwater, the presence of high arsenic contamination in certain KHM sampling sites, and the factors influencing arsenic distribution in the Mekong River sub-region groundwater. These findings provide valuable insights for understanding the water quality and potential risks associated with arsenic contamination in the study area. By providing a clearer understanding of the complex interactions through the results obtained, this study lays the groundwork for further research on the impact of climate change and its effects on the system. Further investigation into the seasonal fluctuations such as the influence on clay layer deposition rate utilising tritium tracers would provide a better insight on the impact of flooding on arsenic mobility especially in highly contaminated area is suggested.
Local and regional policies related to water quality and public health can vary widely depending on the specific area and its unique challenges. Water quality and risk assessment, including water quality monitoring and the evaluation of health impacts, stakeholder engagement, technology transfer, and collaboration – especially regional cooperation – can lead to more comprehensive and effective solutions for resolving the current issue. In addition to that, implementing suitable climate mitigation technology and infrastructure, such as promising treatment technologies like nanofiltration membranes, which achieve a 99.8% arsenic removal rate (Worou, Chen, & Bacharou, 2021) or low-cost hydrotalcite-like compound adsorbent (Kato et al., 2020), is recommended in regions where high arsenic concentrations have been detected. This is to minimise potential health risks within the community.
5. ACKNOWLEDGEMENTS
The authors would like to acknowledge the Asia-Pacific Network for Global Change Research Collaborative Research for Early-Career Scientists Small Grants Programme (CRECS2020-03MY-Seah) for funding this project. Part of the funding was supported by the Gwangju Institute of Science and Technology (GIST) Research Institute (GRI) grant number 140167 in 2022. The authors would also like to thank students in Food Chemistry Lab, Faculty of Science and Technology, International University for their field and lab assistance.